There is no shortage of surprises and twists in the decade-long fight over the control of dominant IP in the CRISPR space. The newest one is the self-revocation of two seminal CRISPR patents in Europe by the team led by two Nobel Laureates Emmanuelle Charpentier and Jennifer Doudna (aka “CVC”). This extraordinary move creates additional uncertainty for companies in the gene editing space trying to navigate the highly complex CRISPR IP landscape and for companies already paying exorbitant licensee fees to these dominant patents in order to secure market exclusivity.
The seemingly suicidal decision appears to be driven by unfavorable preliminary opinions issued by the Board of Appeal of the European Patent Office (the “Appeal Board”), which stated that CVC’s earliest patent filing, intended to protect the Nobel Prize-winning discovery, does not meet the requirements for a patent because it failed to disclose certain key features of the CRISPR technology needed for other scientists to use the technology.
In its 73-page letter accompanying the request to revoke its own patents, the Nobel Laureates’ lawyer explained “The Patentees cannot be expected to expose the Nobel Prize-winning invention protected by the present patent to the repercussions of a decision handed down under such circumstances, when other members of this family (such as e.g., EP 3597749) are still at a stage where the Patentees can procedurally ensure that they will ultimately be fully heard by the Board on all substantive issues.”
Clearly, the Nobel-winning team’s intention was not to surrender its control of dominant IP in the CRISPR space in Europe; rather, it was trying to avoid a final revocation decision by the Appeal Board that could negatively impact the entire patent family and its ability to get new patents in Europe. By self-revocation, CVC is hoping to use pending cases from the same patent family to secure new patents in Europe. To put it bluntly, CVC effectively created a twilight zone between the life and death of its most dominant CRISPR patents in Europe.
CVC’s decision was met with some harsh criticisms by its opponents. One opponent characterized the move as cowardly − “[t]hey are trying to avoid the decision by running away from it”. Another opponent pointed out that CVC’s move “is likely to cause significant delays in terms of certainty for third parties . . . on such commercially relevant subject matter.”
The two patents involved are EP2800811 and EP3401400. Both contain broad claims covering almost any method of modifying a target DNA using a CRISPR-Cas 9 system. These very broad patents originated from a provisional application filed in May 2012 (P1), shortly before the Nobel Laureates published their groundbreaking discovery in the prestigious journal Science in June 2012. Interestingly, the May 2012 provisional left out a key detail − the PAM sequence. This detail was added in a second provisional application filed in October 2012 (P2), after the Science publication, as essential for CRISPR-Cas 9 binding and cleavage.
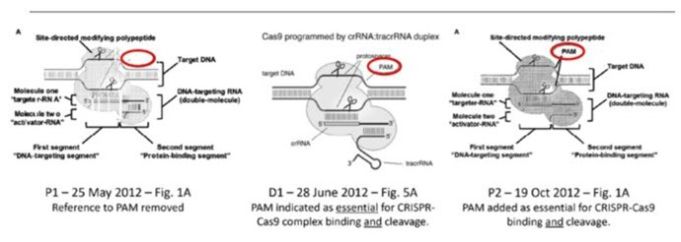
CVC’s opponents argued, and the Appeal Board appears to have agreed, that without knowledge of the PAM sequence and its critical role, scientists in the gene editing space cannot use the CRISPR technology as described in P1. Therefore, P1 does not meet the requirement for a sufficient disclosure of invention and cannot support the later issued European patents, EP2800811 and EP3401400. In other words, EP2800811 and EP3401400 lost their entitlement to the priority date of P1, May 2012. As a result, the Nobel Laureates’ Science paper published in June 2012 (cited as D1 during opposition) becomes invalidating prior art against their own patents.
It is unclear why the PAM sequence was removed from P1 and whether the removal was intentional or accidental. One thing is clear from this saga − no one is above the patent law. Even a Nobel Prize-winning invention must meet the patenting requirements in order to enjoy the protection afforded by the system. One such requirement is to enable the public to practice the patented invention. This stems from the well-known quid pro quo principle of the patent system: Patentee makes a full and enabling disclosure to the public to allow others to build on their invention in exchange for limited monopoly.
In theory, CVC’s self-revocation of EP2800811 and EP3401400 may somewhat insulate other family members from a negative final decision. However, it will not fix the problem of P1. Other family members including potential new patents issuing from the pending cases will likely suffer from the same defect as EP2800811 and EP3401400. More importantly, self-revocation will not insulate the public from forming its own opinion. The fact that the Nobel team would go so far as to revoke its own seminal patents in the wake of a board opinion will cast long-lasting doubt over the strength of its IP position in the gene editing space.
